
Working out the shapes of molecules is a fascinating topic in chemistry; stereochemistry; that is the study of the three-dimensional (3D) arrangement of atoms, molecules and ions in space is the term which is often used when discussing the shapes of
molecules. Molecules
come in all sorts of shapes and sizes; from small molecules such as
water to very large biological
polymers
such as proteins and DNA. The shape of molecules is perhaps most important in the chemical reactions that
take place in living organisms where a small alteration in the shape of an enzyme or a drug molecule can have dramatic
effects on its action or its side effects.
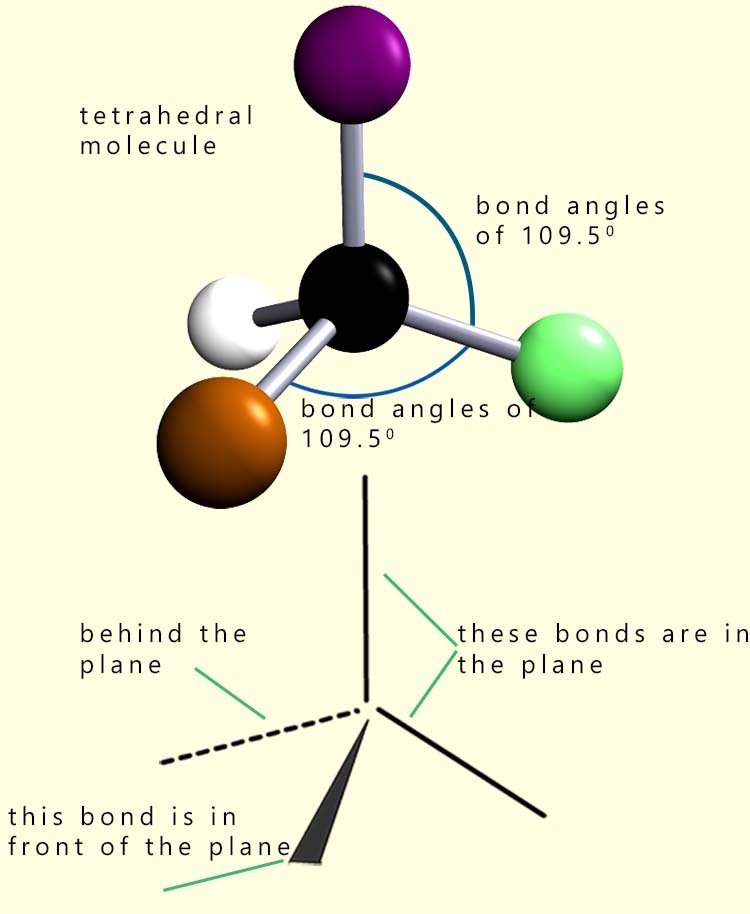
The overall shape of a molecule is determined by its bond angles; these are the angles between the lines representing the covalent bonds between the nuclei of the atoms that make up the molecule. The molecule shown opposite has a central black atom surrounded by 4 other atoms. The shape of this molecule is described as tetrahedral. All the bond angles in the molecule are all 109.5°.
To draw 3d molecules such as this tetrahedral one we use dotted or dashed lines to represent covalent bonds which are behind the plane and a dark shaded triangular line to represent covalent bonds sticking out in front of the plane. Solid lines show bonds which are in the plane. You can see in this tetrahedral molecule that 2 of the covalent bonds are in the plane; one is behind and one is in front. This is shown in the image opposite.
The VSEPR model is the one we are going to use to work out the shapes of different
molecules. Its name is a
bit of a mouth full but it is very easy to apply and working out the shapes of molecules using this model is
very straight forward. As the title suggests we need to consider the electrons in the valence shell (the outer shell); these are after all the electrons
that are involved in bond formation. We already know that these electrons pair up to form
covalent
bonds; which involve the sharing of a pair of electrons. Recall that the two electrons in a covalent bond are attracted to the nuclei of the
two atoms involved in forming the covalent bond and it is this attraction that holds the two electrons in the covalent bond in place.
However the pair of
electrons in a covalent bond will repel other electrons involved in other covalent bonds around the central atom in a molecule. As a
result of this repulsion the electron pairs will get as far apart as possible while still retaining their
distance from the nuclei.
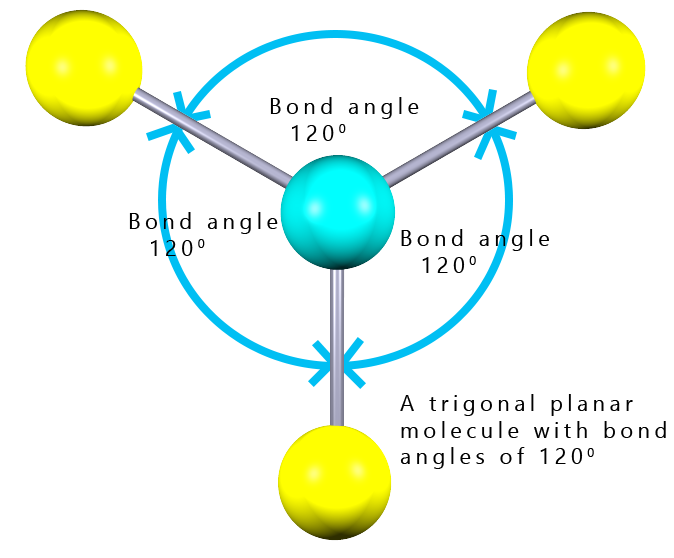
In the example shown opposite we have a molecule with the formula AB3; where there are 3 covalent bonds around the central blue atom. Each of these covalent bonds contains a pair of electrons and since they are all negatively charged they will repel each other and try to get as far apart in 3d space as possible. So how can three electron pairs arrange themselves in space to get as far apart as possible? The answer is to form a flat (planar) molecule with bond angles of 120° The shape of this molecule is described as trigonal planar.
The shapes of molecules with single covalent bonds between the atoms can be easily predicted using the VSEPR model. The shape of a molecule is basically determined by the number of bonding pairs of electrons and lone pairs of electrons around the central atom. Now being negatively charged these bonding pairs and lone pairs of electrons will obviously repel each other as much as possible in 3d space. So working out the shape of a particular molecule then is simply a case of working out how these bonding pairs and lone pairs of electrons orientate themselves in such a way as to reduce this repulsion between them.
The image below shows dot and cross diagrams and models for the structure of three molecules you are probably very familiar with; ammonia, water and methane. From the image you can see that:
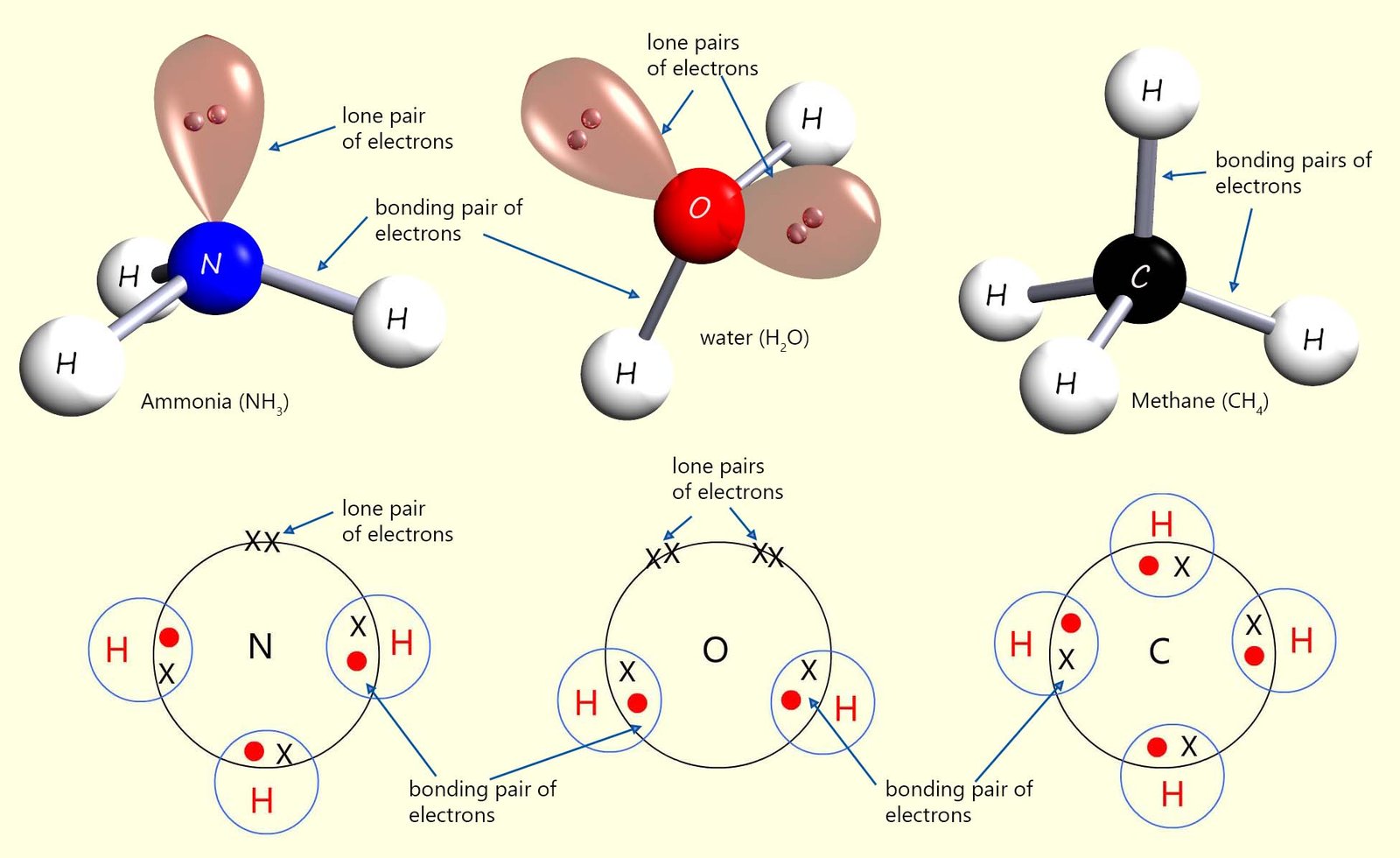
Now each of these molecules has 8 electrons in its valence or outer shell, 2 electrons in each bonding pair and 2 electrons in each lone pair.
 The lone pairs and bonding pairs of electrons all have a negative charge and will obviously repel each other; so what shape or orientation can they take in order to try and reduce this repulsion as much as possible? Using the VSEPR
model we can say that these lone pairs and bonding pairs of electrons can reduce repulsion between them by forming a tetrahedral shaped molecule, as shown in the image. If you look carefully you will see that each of the molecules above; despite first appearances all have the same basic shape; that is a tetrahedral shape.
The lone pairs and bonding pairs of electrons all have a negative charge and will obviously repel each other; so what shape or orientation can they take in order to try and reduce this repulsion as much as possible? Using the VSEPR
model we can say that these lone pairs and bonding pairs of electrons can reduce repulsion between them by forming a tetrahedral shaped molecule, as shown in the image. If you look carefully you will see that each of the molecules above; despite first appearances all have the same basic shape; that is a tetrahedral shape.
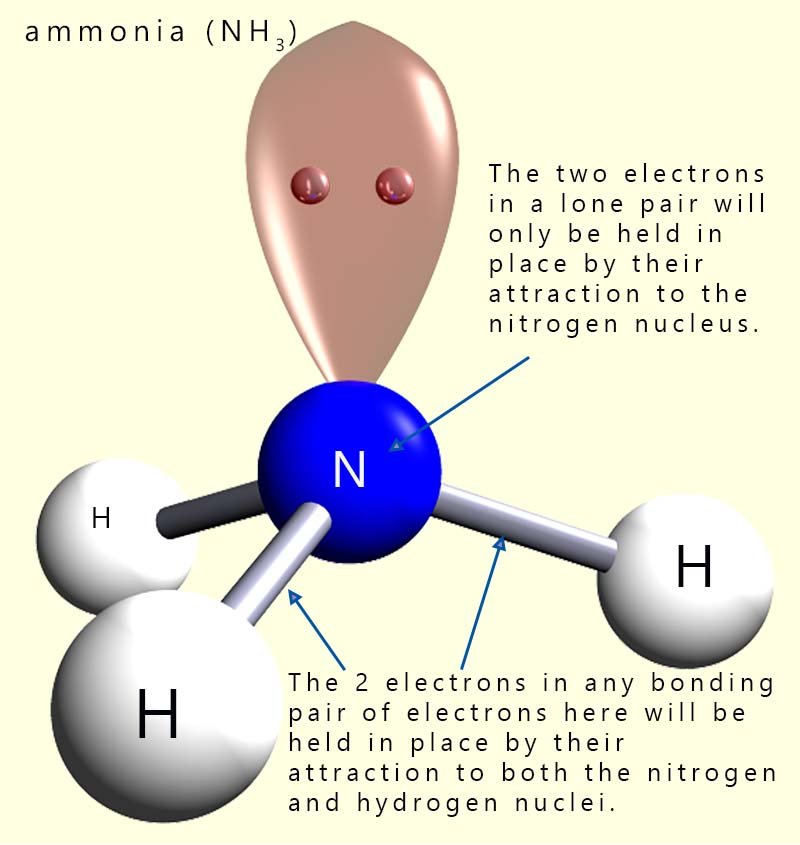
Now in a bonding pair the 2 electrons which are shared in the covalent bond are held between the two nuclei of the atoms involved in forming the covalent bond, while in a lone pair of electrons the two electrons are being held in place by their attraction to only one nucleus. This means that a lone pair of electrons will take up more space than a bonding pair of electrons. The presence of lone pairs of electrons in a molecule will affect the bond angles in any molecule which contains them and it is important that you locate them if they are present in order to determine the overall shape and bond angles present in the molecule.
For molecules that contain more than one lone pair then this will have a further effect on the bond angles present, we can summarise the repulsion between bonding pairs and lone pairs of electrons as:
| lone pair ↔ lone pair | is greater than | lone pair ↔ bonding pair | is greater than | bonding pair ↔ bonding pair |
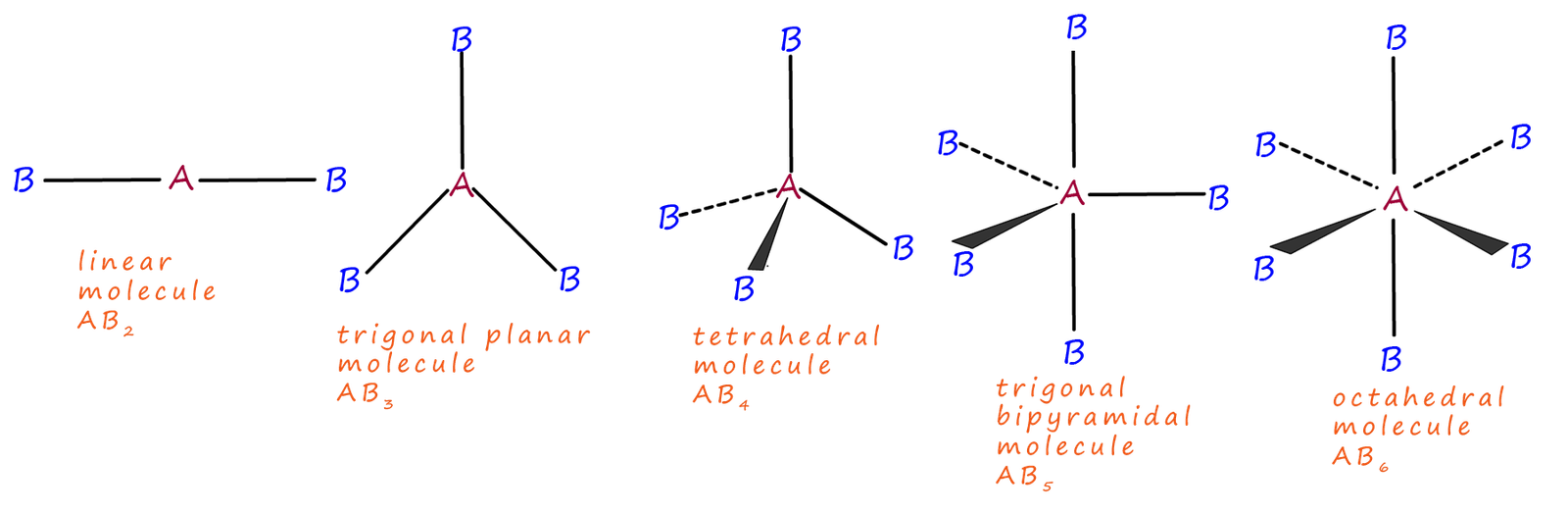
However before we start to work out the shapes of various molecules it would help if we looked at the common shapes which are found in many molecules. You should make yourself familiar with these basic shapes and bond angles as they are the basis for all of the molecular shapes you are likely to be asked to work out. The basic shapes of common molecules are based on those shown in the table below:
The basic shape of a molecule will be determined largely by the number of electron pairs both bonding and lone pairs around the central atom in a molecule. These electron pairs will repel each other and try to get as far apart as possible in 3d space. The table below shows the basic shapes of molecules with up to four electron pairs around the central atom in a molecule and also the bond angles between these electron pairs.
| Number of electron pairs | Shape of molecule | Name of molecular shape |
|---|---|---|
| 2 | 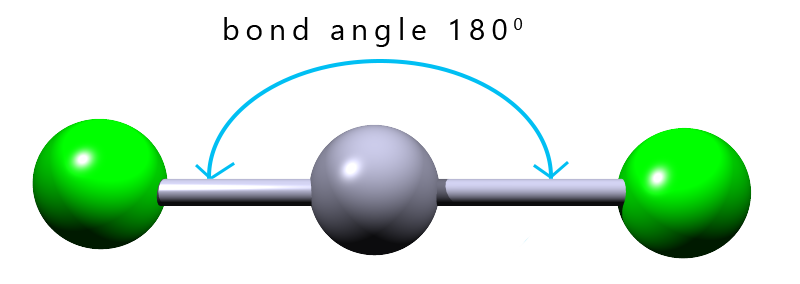 |
linear |
| 3 | 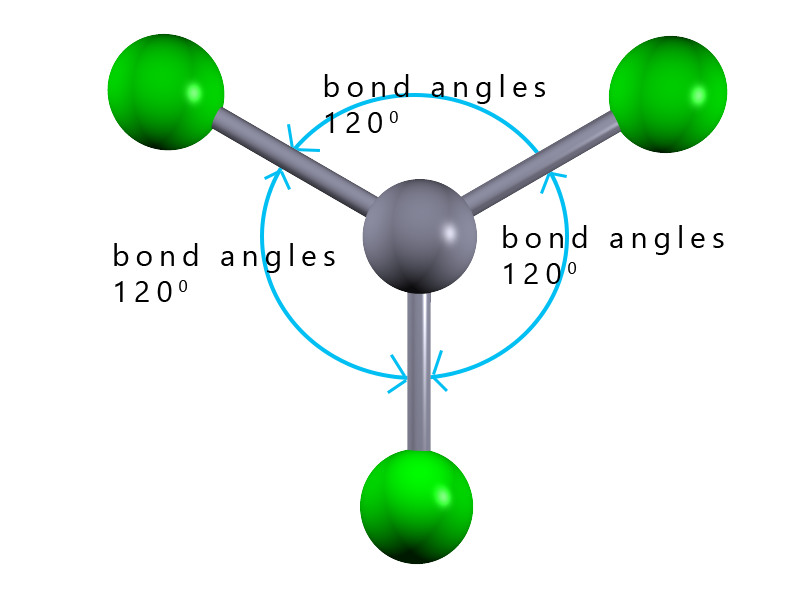 |
trigonal planar |
| 4 | 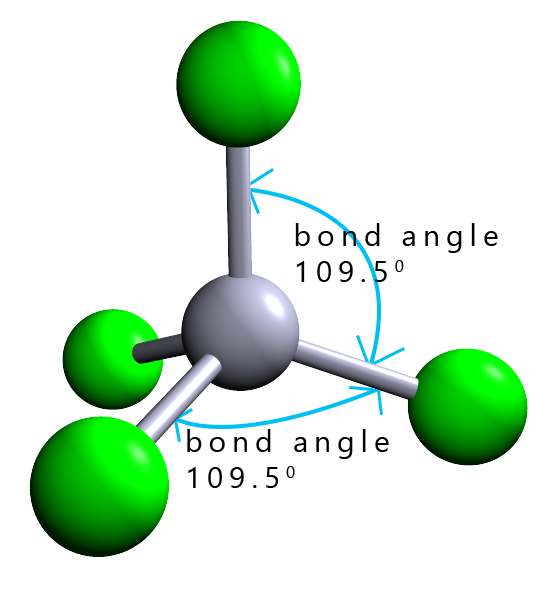 |
tetrahedral |
The tetrahedral molecule has 4 bonding pairs or 8 electrons around the central atom. In gcse chemistry we learned that 8 electrons around a central atom was the maximum number allowed to give a stable octet of electrons. However elements in period 3 of the periodic table and above can have an expanded valence shell which allows them to have five or six pairs of electrons around the central atom. The shapes and the bond angles in these molecules are shown in the table below.
| Number of electron pairs | Shape of molecule | Name of molecular shape |
|---|---|---|
| 5 | 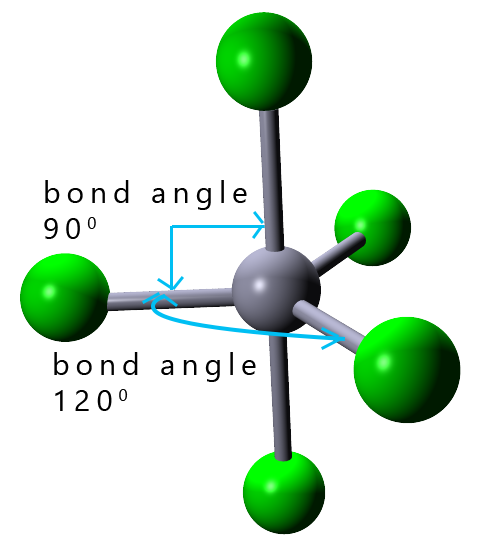 |
trigonal bipyramidal |
| 6 | 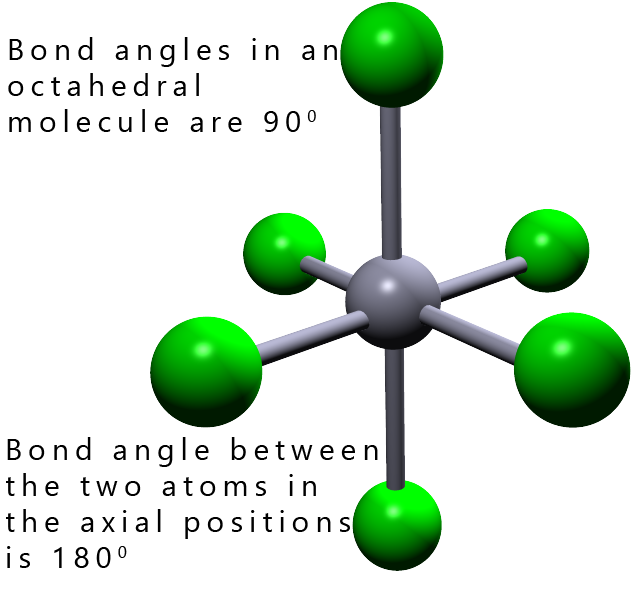 |
Octahedral |
As a quick review to test your knowledge of bond angles present in molecules try the activity below:
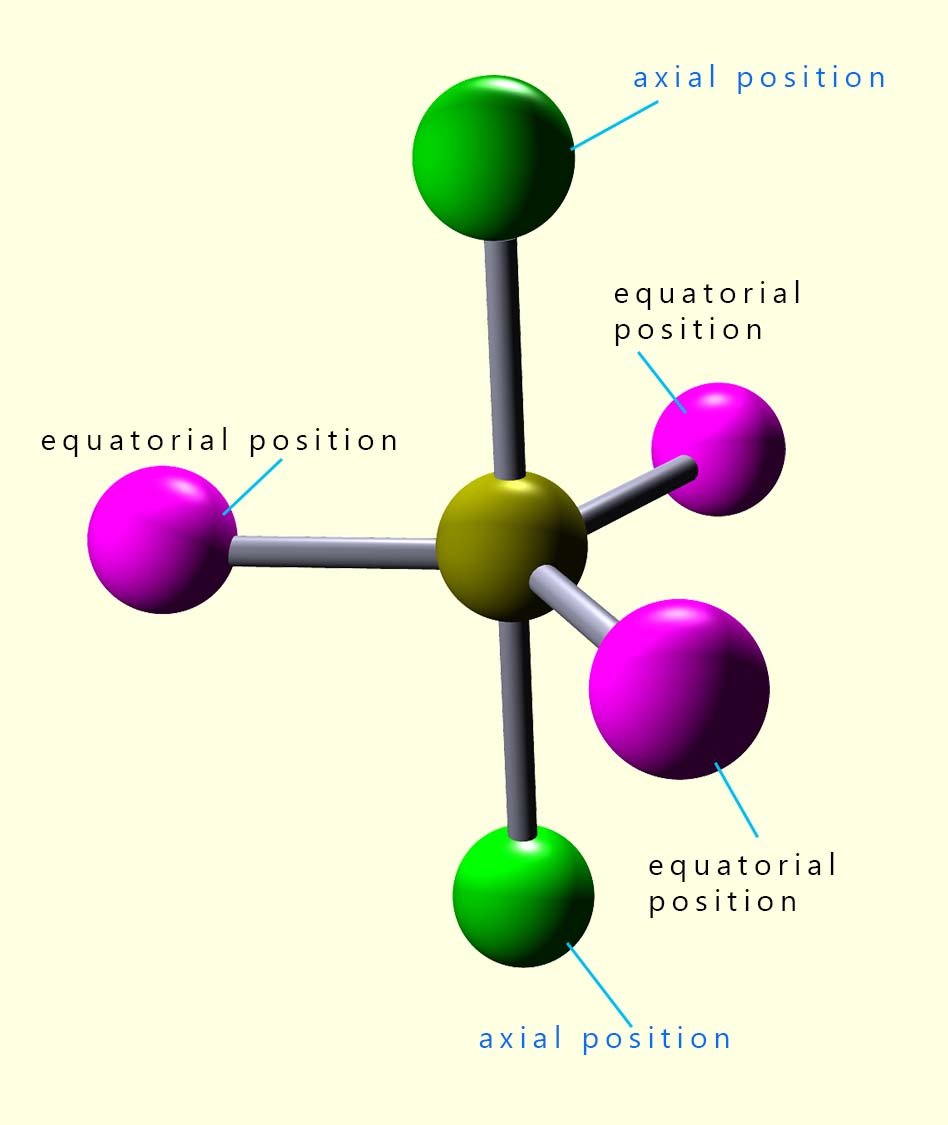
In small molecules with up to four electron pairs; that will be linear, trigonal planar and tetrahedral shaped molecules and also larger octahedral molecules all the bond angles in these molecules are the same. If you built models of these molecules and then flipped then on your desk no matter which way the molecule landed it would always look the same, simply because all the bond angles are the same in all these molecules. In an octahedral molecule the bond angles between adjacent atoms are all 90° while the bond angle between any atoms opposite or directly across from each other is 180°. In trigonal bipyramidal molecules there are two different positions; called the axial and equatorial positions. These two different positions are shown in the image opposite:
In a molecule with a trigonal bipyramidal shape there are two different environments in which the bonded atoms can be in when they
surround the central atom. These two positions are called the equatorial and the axial positions. In the image opposite there are 3 atoms around the middle part of the trigonal bipyramidal molecule shown. They are separated from each other by bond angles of 120°. These atoms are described as being in an equatorial position.
There are also two atoms shown in green which are both at 90° to the equatorial atoms. These atoms are said to be in an axial position.
Test yourself on the basic shapes of molecules; the table below is a repeat of the one above except the shape name and bond angles are missing. For each molecule in the table record or predict the shape and bond angles before clicking the show answer button- don't cheat by scrolling up to have a quick look!!!
| Number of bonding pairs | 3D model | Shape name | Bond angle(s) | Example |
|---|---|---|---|---|
| 2 | 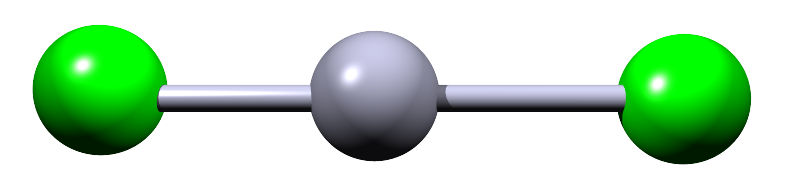 |
Beryllium dichloride (BeCl2) | ||
| 3 | 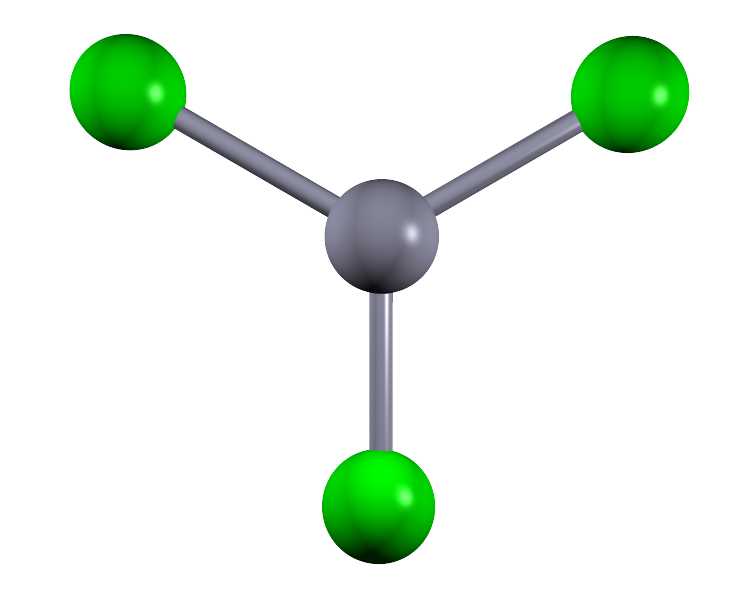 |
Boron trifluoride (BF3) | ||
| 4 | 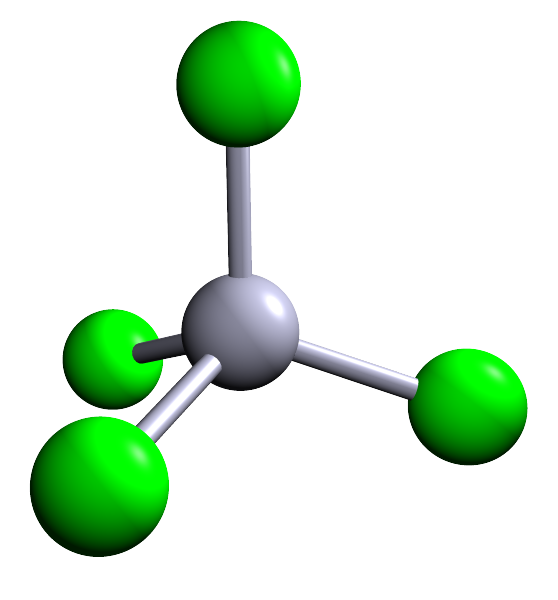 |
Methane (CH4) | ||
| 5 | 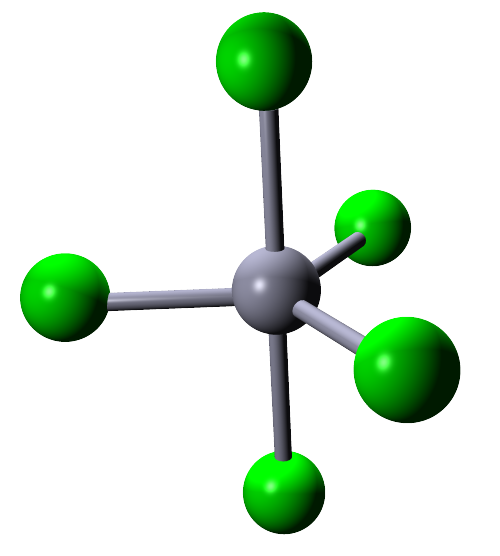 |
Phosphorus pentachloride (PCl5) | ||
| 6 | 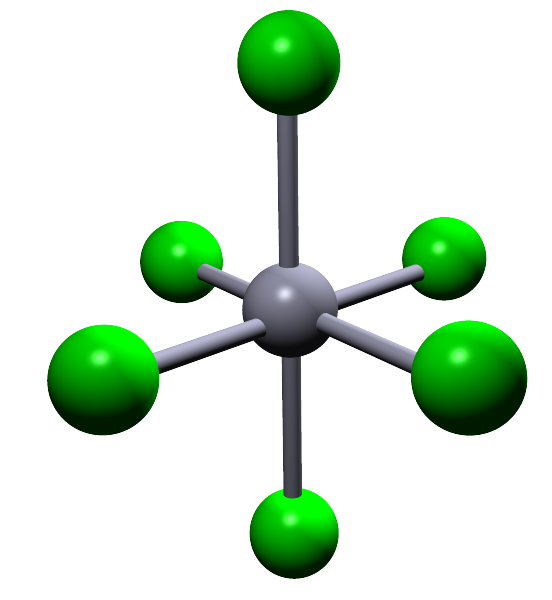 |
Sulfur hexafluoride (SF6) |